Metalloneurochemistry
|
|
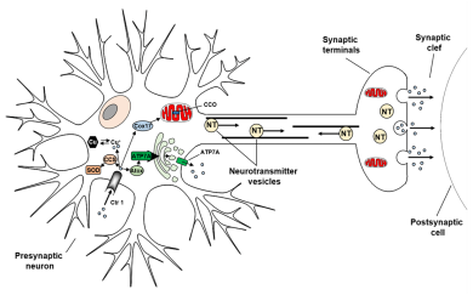
The central nervous system contains high concentrations of transition metals (such as zinc, copper and iron), where they play essential structural, catalytic and signalling roles. As a consequence their uptake and distribution are tightly regulated to meet cellular requirements.
Metal dis-homeostasis however appear to underlie a number of neurodegenerative amyloid disorders, such as Alzheimer's disease, Parkinson's, and Prion disorders, characterized by protein misfolding and aggregation (amyloid-beta peptide, alpha-synuclein, and prion protein, respectively). Aberrant metal-protein interactions with these peptide/proteins contribute to disease progression by modulating the formation and toxicity of soluble and insoluble amyloid oligomers and their aggregates, and by radical production through redox-cycling from these metal-protein complexes.
Metal dis-homeostasis however appear to underlie a number of neurodegenerative amyloid disorders, such as Alzheimer's disease, Parkinson's, and Prion disorders, characterized by protein misfolding and aggregation (amyloid-beta peptide, alpha-synuclein, and prion protein, respectively). Aberrant metal-protein interactions with these peptide/proteins contribute to disease progression by modulating the formation and toxicity of soluble and insoluble amyloid oligomers and their aggregates, and by radical production through redox-cycling from these metal-protein complexes.
Metallochemistry and biophysics of amyloid disorders
In our laboratory we explore the coordination chemistry properties of amyloidogenic peptides and proteins involved in the neurodegenerative processes, and characterize the role of transition metal ions in triggering peptide/protein oligomerization, radical production, membrane interaction and pore formation.
In our laboratory we explore the coordination chemistry properties of amyloidogenic peptides and proteins involved in the neurodegenerative processes, and characterize the role of transition metal ions in triggering peptide/protein oligomerization, radical production, membrane interaction and pore formation.
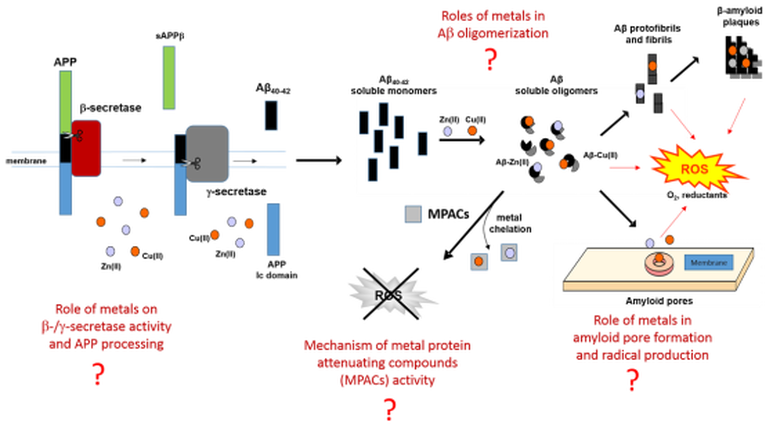
In parallel we investigate the reactivity and redox properties of metal chelators and metal-protein attenuating compounds (MPACs), as an emerging class of compounds targeting aberrant metal-protein interactions in the brain. In particular, we are interested in studying the structure and reactivity of a brain metalloprotein named metallothionein-3 (MT-3, also known as neuronal growth inhibitory factor) which show protective effect as endogenous "MPAC".
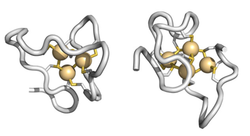
MT-3 binds with high affinity monovalent and divalent metal ions into two metal-thiolate clusters (figure below) and can efficiently efficient scavenge "free" and tightly bound Cu(I)/(II) through metal-swap reactions, thereby controlling aberrant metal-protein interactions and abolishing free radical production through redox silencing.
The overall goal of this line of research is to elucidate the chemistry of metals in amyloidogenic processes and establish the rationale for the development of metal chelating compounds preventing the amyloid cascade.
MT-3 binding through the metal-thiolate clusters.